Reproduction
Reproduction

Sexual reproduction
Like many marine invertebrates, corals have a two-phase life cycle, a planktonic larval phase and a sessile adult phase, where most species live attached to the reef. Also like most marine invertebrates, corals have only minor control of dispersal; they can control the time of release of larvae and they can control the time the larvae remain afloat, or at least the physiological timing of larval development and period of competency before settlement from the plankton and metamorphosis are necessary. Periods of larval competency and times to settlement and metamorphosis can range from as little as several hours for some brooding coral species to several months for the longest-lived larvae of some broadcast spawning species, with obvious implications for dispersal.
For the reproductively-mature adult corals, timing is doubly important because males and females cannot move into reproductive contact: species which spawn must release their gametes into the water simultaneously. As colonies may be a long way apart, this release must be both precisely and broadly synchronised. This is done in response to environmental cues, notably the phase of the moon, sea temperature and day length, responses which have a wide array of options. Many of the broadcast-spawning coral species time their reproductive cycles to coincide with the period following the full moon. This occurs in Spring on Australia’s Great Barrier Reef, in Autumn on Australia’s west coast, and at other times elsewhere. Spawning is typically initiated in the late afternoon – early evening, although the precise spawning periods of different coral species occur on different days following the moon.
Some species may have a ‘split-spawning’ where reproduction occurs over two successive lunar cycles in a particular year. Additionally, corals on reefs developed under different sets of environmental conditions may spawn on a different moon, as occurs on Australia’s Great Barrier Reef, where corals on nearshore reefs typically spawn one month earlier than those living further offshore on reefs of the mid- and outer-continental shelf. In equatorial regions, spawning for many species appears less synchronized, occurring over longer time periods than those of the ‘mass spawning’ episodes of higher latitudes.
For larval brooding species, including members of the Indo-Pacific general Isopora and Seriatopora, fully developed planula larvae are released from the parent more regularly, over a period of several months, again often timed to coincide with a particular lunar phase. These can settle very rapidly, within several hours of release, fostering local abundance. Additionally, some species successfully combine brooding with long-distance dispersion by attaching to floating objects (rafting). Others have planulae that have both rapid settling and long-distance dispersal capability. Yet others release gametes as well as brood planulae, including some species of Pocillopora.
Hermaphroditism and gonochorism
About three-quarters of all zooxanthellate corals are hermaphrodite; the remainder are gonochoric, having separate male and female colonies, including species of Porites, or (in solitary species) separately sexed individuals, as in Fungia. The sexuality of corals — whether they are hermaphrodite or separately sexed — tends to be generally consistent within species and genera, although there are exceptions. There is sometimes also geographic variation within species.
About three-quarters of all zooxanthellate corals spawn eggs and sperm for external fertilisation rather than brood planulae within their bodies after internal fertilisation. These include the major Indo-Pacific genera Acropora and Montipora. Spawning is associated with high numbers of eggs and, when fertilisation is successful, planulae, while brooding results in fewer, larger and better developed planulae. This tends to be variable, even within genera. Because of numbers, release of gametes into the ocean more typically facilitates long-distance dispersion and thereby the creation of genetic links between one reef region and another. Such dispersal is contingent on the surface ocean currents flowing across the natal reef at time of spawning, a highly variable phenomenon in both space and time. In some cases, larvae may be retained in localized eddies or gyres on their natal reef. In others, currents may carry larvae tens, hundreds or even thousands of km to distant reefs. This phenomenon is best observed in the large unidirectional current systems of the east and west coasts of Australia (East Australian and Leeuwin Currents respectively), and the Kurishio current that flows northwards through the Ryukyu Island chain towards Japan.

Figure 1. Spawning in hermaphroditic corals. (a) An Acropora showing egg and sperm bundles that have moved to the mouths of the polyps just prior to spawning. Great Barrier Reef, Australia. Photograph: Valerie Taylor. Figure 1a.
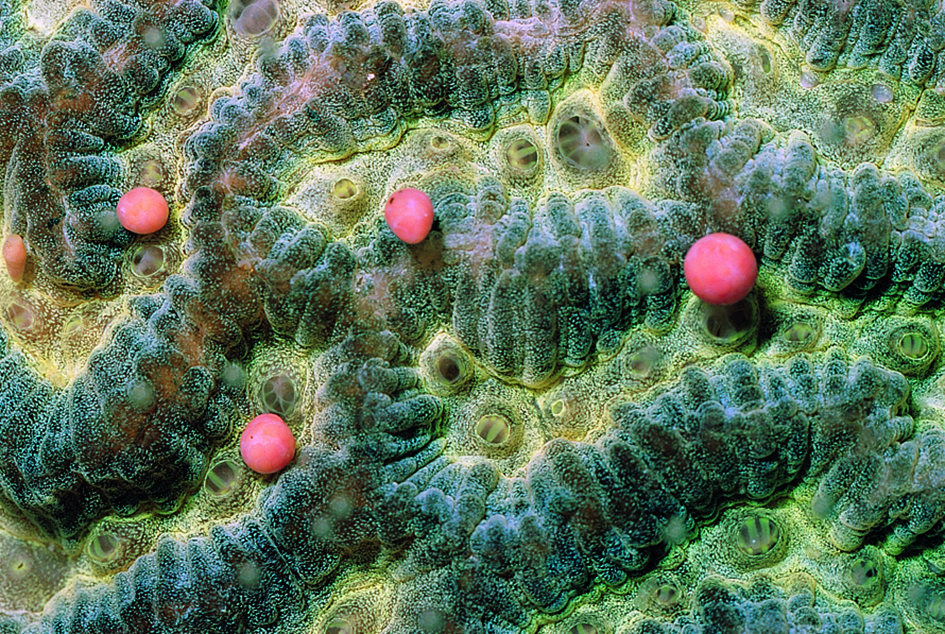
(b) A Platygyra with egg and sperm bundles that have just been released. Kuwait. Photograph: Peter Harrison. Figure 1b.
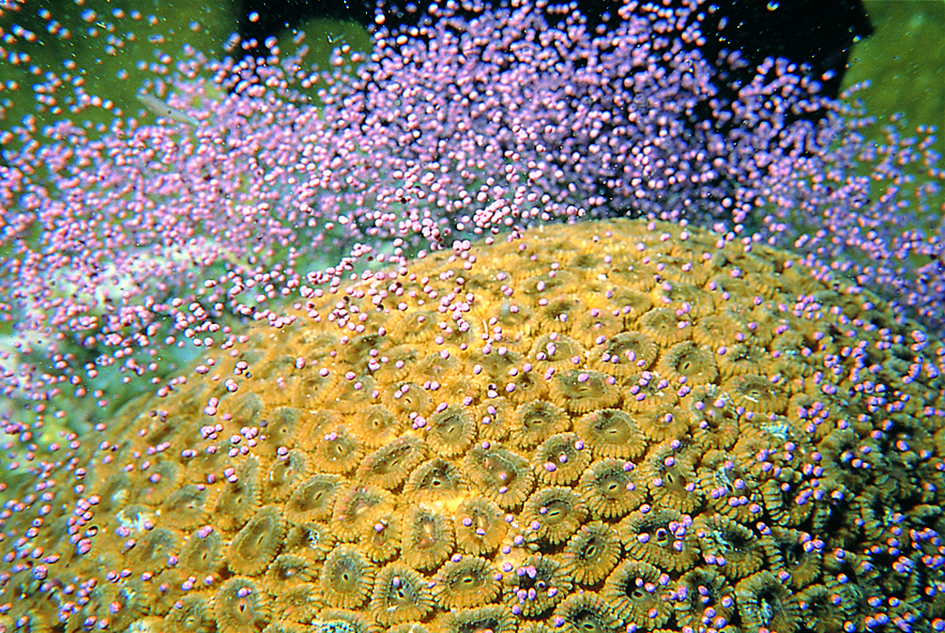
(c) A Favia showing the upward-moving shower of egg and sperm bundles that can come from a single colony. Lord Howe Island, south-eastern Australia. Photograph: Peter Harrison. Figure 1c.
In contrast, brooding corals can readily concentrate planulae close to the parent colony, with settlement occurring rapidly, often within several hours of release, as can occur in species of Isopora. Reproductive mode may therefore involve finding balances. These include balancing local abundance (by having planulae that settle rapidly) and long-distance dispersion, self-fertilisation (internally or within egg and sperm bundles) versus outcrossing, and within-species fertilisation versus 'hybridisation'. When transferred to biogeographic scales, these balances affect the genetic composition of species and how they form geographic patterns (see 'Evolution'). As a result, a single species of coral may have different reproductive modes in different geographic regions.
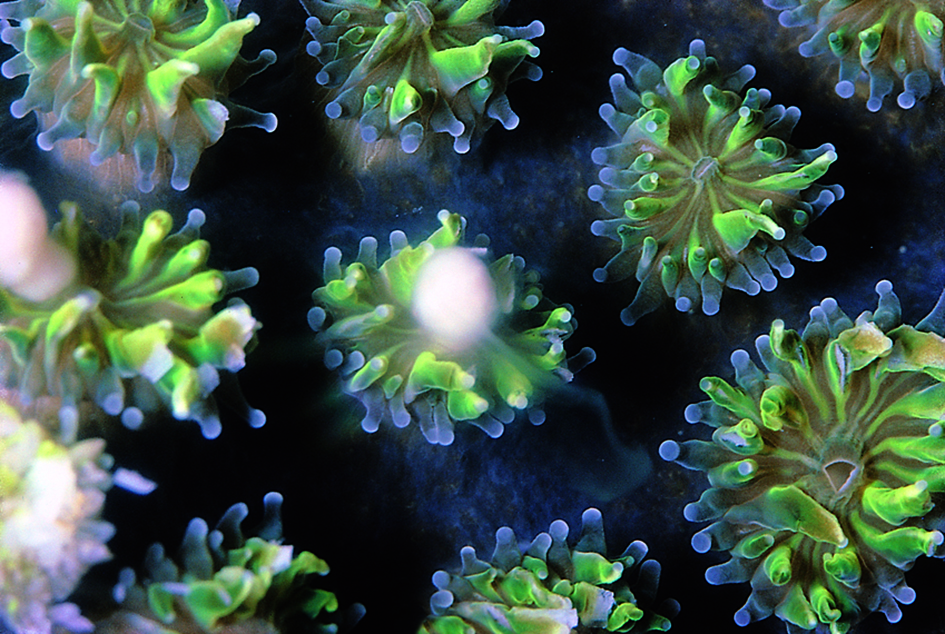
Figure 2. Spawning in separately sexed corals. (a) Galaxea. The male has a white globule of sperm and undeveloped eggs. Great Barrier Reef, Australia. Photograph: Peter Harrison. Figure 2a.

(b) Galaxea. The female only has eggs. Great Barrier Reef, Australia. Photograph: Peter Harrison. Figure 2b.

(c) Fungia. The male is releasing a smoke-like cloud of sperm. Great Barrier Reef, Australia. Photograph: Peter Harrison. Figure 2c.

(d) Fungia. The female is releasing a stream of eggs which are not clustered into bundles. Great Barrier Reef, Australia. Photograph: Peter Harrison. Figure 2d.
Synchrony
The long-term control of spawning (control of the maturation of gonads) may be set by temperature, day length and/or rate of temperature change (either increasing or decreasing). The short-term (getting ready to spawn) control is usually lunar. The final cue (release of spawn) is usually the time of sunset. There are many variations on these controls, probably because synchrony is usually linked to whatever environmental cues work best within a given region. Cues may also be biological as well as physical and synchrony by chemical messengers may not only involve corals, but a host of other marine life as well.

Figure 3. Egg and sperm masses on the ocean surface. (a) A concentration of egg and sperm bundles on the surface. Some are breaking apart and some are starting to develop into planulae. Great Barrier Reef, Australia. Photograph: Valerie Taylor. Figure 3a.

(b) Reproductive material forming slicks floating over reefs. These huge slicks gradually break up into countless millions of planulae. Depending on current patterns, this may leave little time for the development and settlement of planulae before they are transported away from the reef of origin. Great Barrier Reef, Australia. Photograph: Bette Willis. Figure 3b.
Brooding species, which release planulae and not gametes, can store unfertilised ova for weeks and thus require less synchrony for fertilisation. Spawning species require synchrony within a time frame of hours. This regional synchrony varies geographically: for example, on the Great Barrier Reef, spawning occurs in October/November, in Western Australia it occurs in March and in Japan in June/July. These differences suggest that synchrony, if under a genetic control that is not modified by local environment, could be a barrier to effective long-distance dispersal: a planula larva could successfully make a long journey, only to develop into a colony which is reproductively isolated (spawns asynchronously) from other colonies in the region if this is under partial genetic control.

Figure 4. Development of planulae from fertilised eggs. (a) A mixture of eggs and developing embryos, still part of a slick on the ocean surface. Great Barrier Reef, Australia. Photograph: Peter Harrison. Figure 4a.
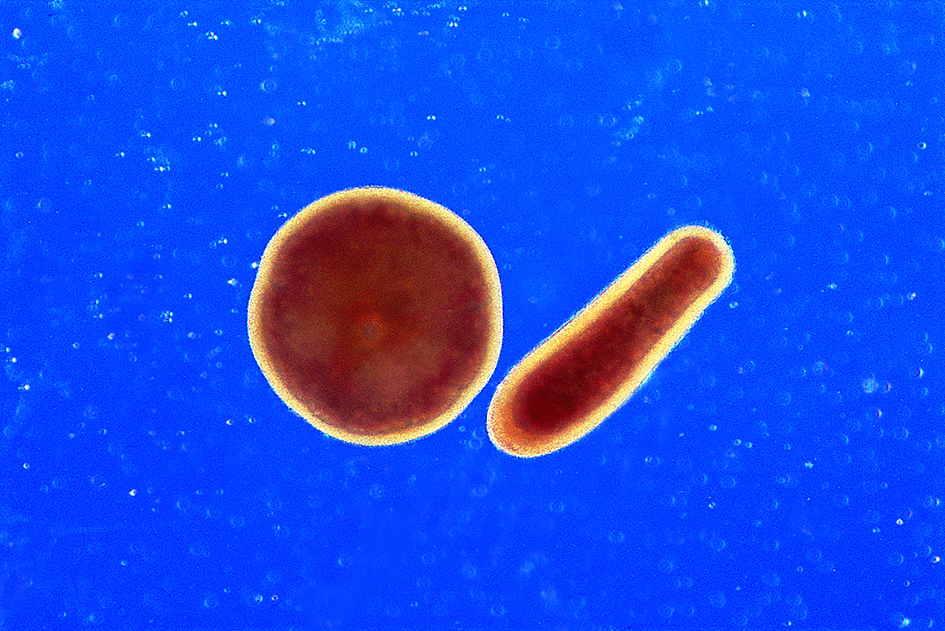
(b) Planulae of Acropora. Planula larvae typically change shape from spherical to elongate as they develop. The outer surface is covered with cilia allowing some motility, the interior is darkened with zooxanthellae. Great Barrier Reef, Australia. Photograph: Peter Harrison. Figure 4b.
Hybridisation
In most major coral regions, not only do different colonies of the same species synchronise their spawning, but colonies of different species, and indeed genera, have the same synchrony. The outcome, in many regions, is 'mass' spawning. When mass spawning occurs, the ocean surface becomes a soup of genetic material creating endless possibilities for cross fertilisation. The extent to which hybridisation occurs — that eggs of one species are fertilised by the sperm of another — is not known. What is known is that different species within the same genus (and rarely between species of different genera) can readily hybridise and that the progeny can be normal-looking corals. Examples include crosses between different species of Acropora, notably the production of the Caribbean hybrid species Acropora prolifera from Acropora cervicornis and Acropora palmata. Successful crosses between genera include those from Platygyra and Leptoria.
This is not what might be expected because if all species within a genus were to hybridise in this way, distinctions between the hybridising species would disappear and there would only be one species. In such a case, maintenance of species distinctions could only be by geographic separation. Geographic separation greatly affects the genetic composition of species, but clearly there must be other mechanisms for maintaining species distinctions within a single region. In many groups of plants and animals, there are behavioural, genetic, or anatomical barriers to hybridisation. Under conditions of mass spawning, such barriers might include reduced fertility or reduced capacity for hybrid survival. They may also include mechanisms that mask genetic change, such as a small number of genes being responsible for major morphological outcomes. Whatever the reality, large amounts of geographic space, evolutionary time, and rare events matter enormously when it comes to creating change. With corals, genetic exchange between one species and another clearly occurs. Over vast amounts of space and time, this creates the interacting dynamic geographic patterns within and between species that we observe today.
Dispersal
An important aspect of coral reproduction, and one that underpins biogeographic patterns, concerns the capacity of corals to undertake extended ocean voyages. It is now known that planulae of at least some species can spend months being transported by currents and still be competent to settle. It is likely that the planulae of most species of coral make long-distance journeys and probably do so frequently. The likelihood of survival once a distant destination is reached is extremely small, but again, rare events are likely to be the events that matter, representing a form of 'sweepstakes' dispersal. Planulae can live on the ocean surface for long periods of time because they have zooxanthellae to provide nutrients. They may even undergo partial metamorphosis while afloat. In some instances, juvenile and adult colonies may also disperse on floating objects including pumice. This 'rafting' of colonies is probably an important mechanism of dispersal in some species, especially of Pocillopora, Stylophora and Seriatopora.

Asexual Reproduction
Asexual reproduction also affects the distribution and abundance of many species. Parts of branching corals are commonly scattered by storms only to re-grow as a multitude of new colonies. Large massive colonies of Porites also episodically form cloned ‘daughter colonies’ following isolation and dislodgement of basal parts of the parent colony, a process driven by shading, bio-erosion and wave energy. This does not in itself aid long distance dispersal, but it may greatly enhance the reproductive output, hence the genetic impact, from the settlement of a single planula larva. There are many other mechanisms of asexual reproduction that may play the same role. These include the formation of satellite colonies in Goniopora, the 'bail-out' of polyps as a result of environmental stress, the expulsion of fully developed polyps in place of planulae, the budding of acanthocauli in fungiids and other corals, and autotomy in Diaseris.

Figure 5. Aquarium and field studies are used together to study coral reproduction. (a) A polyp of Oculina with clearly visible calcareous stalk is being released from the parent colony. Mediterranean Israel. Photograph: Amikam Shoob. Figure 5a.

(b) Oculina polyps settle and grow into new colonies. Mediterranean Israel. Photograph: Amikam Shoob. Figure 5b.

An acanthocaulus of Fungia. These juveniles develop long stalks. The stalks are broken by boring sponges, releasing the tiny discs as free-living corals. Great Barrier Reef, Australia. Photograph: Valerie Taylor. Figure 6.

Other options
Other aspects of reproduction and colony formation may have biogeographic and evolutionary impacts. One is the possibility that polyploidy (the possession of more than two entire chromosome complements) occurs in corals just as it does in many groups of plants and some groups of animals. Another is the possibility that mutations in body cells can be transferred to sex cells by the formation of cancerous growths or ‘neoplasms’.

A colony of Platygyra with a neoplasm or cancerous growth. Neoplasms are found in most coral species and they can be reproductive, just like the rest of the colony. Although neoplasms originate from somatic (non sex cell) mutations, they may well be a source of an instant new species. Bali, Indonesia Photograph: Charlie Veron. Figure 7.
Neoplasms, which typically consist of many polyps that are presumed to have grown from a single mutant-containing cell, are common in corals and, in theory at least, raise the possibility of instant new species via sexual reproduction by the mutant polyps. Another type of colony formation occurs when spat or planulae of different genetic origin fuse to form post-fertilisation hybrids or ‘chimeras’. It is common for colonies to form from two or more original spat that fuse as they grow. Similarly, planulae can grow from two or more original eggs that fuse in early development. The outcome, in both cases, is a chimera (a colony that is not what it seems) and these may be post-fertilisation ‘hybrids’ if developed from eggs of different species. Chimeras of many different types (reflecting differing levels of cell integration) are known in many groups of plants and in some groups of animals. However, unless there has been some sort of nuclear DNA fusion or polyploid formation, their reproductive output, if any, will be one or other of the parent species.
The Scleractinia are an ancient group of organisms and, as with many groups of plants, do not have close genetic control over morphological characteristics. This allows them to have a wide range of life-cycle options in reproduction and dispersal and hence biogeographic and evolutionary change. Both reproduction and dispersal are closely linked to concepts of what species are. Corals, like many major groups of plants, have fuzzy species boundaries: species are not single reproductively isolated units and, because of biogeographic variation, there are no essential distinctions between species, subspecies and hybrids. All such taxonomic units are parts of interconnected genetic patterns, created by ocean currents, and forever changing.


