Evolution

Evolutionary history
Scleractinians were preceded in geological history by a range of other reef building organisms discussed further in Reefs. The first organisms that might be called scleractinians are known from Palaeozoic fossils found in China and Scotland, but the earliest proliferation of organisms that were clearly ancestral Scleractinia are Middle Triassic and consisted of at least seven, but possibly nine, suborders. These corals did not build reefs; they were small solitary or phaceloid organisms of the shallow Tethys Sea of southern Europe and Indo-China.
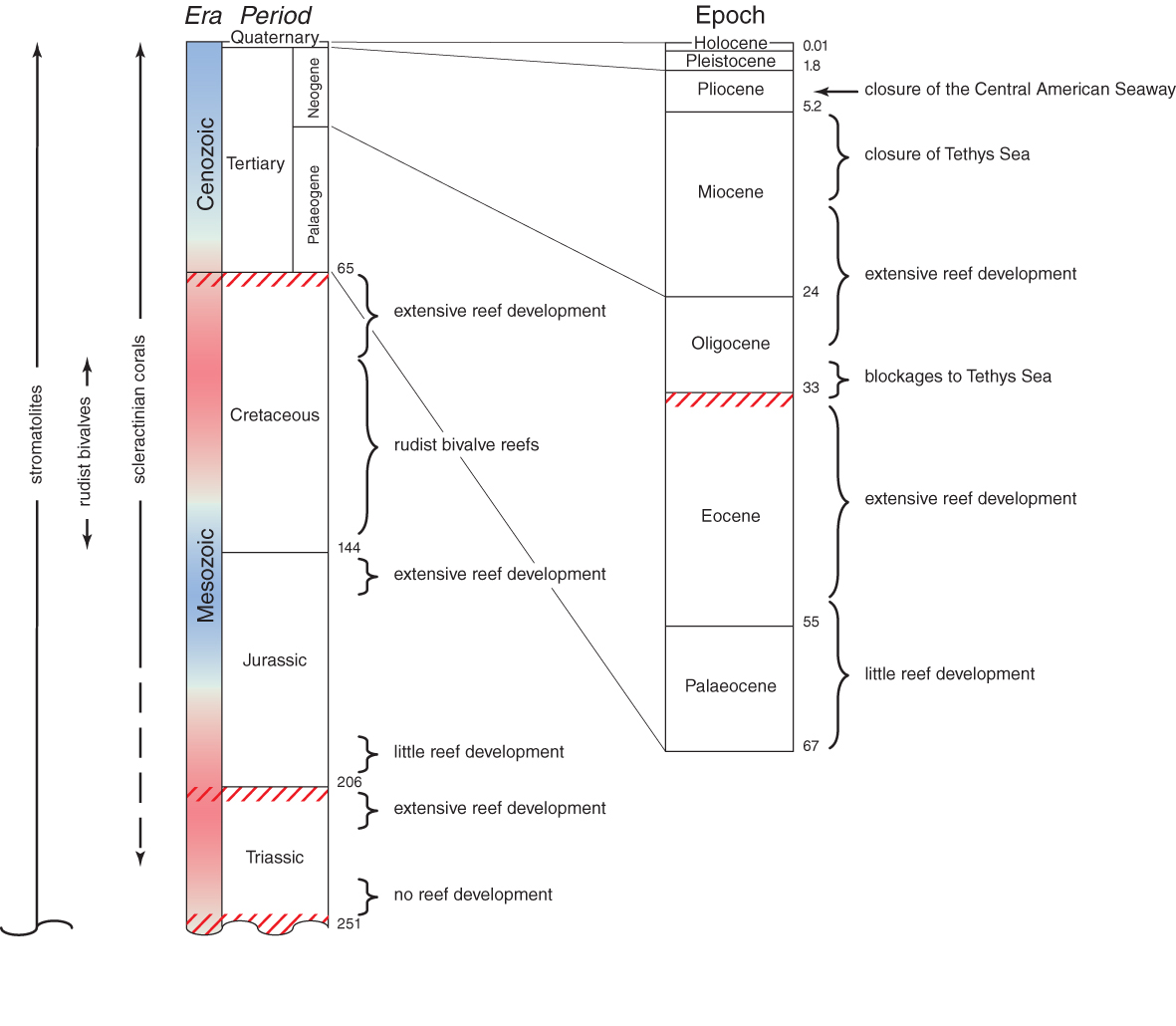
Mesozoic and Coenozoic geological time chart. Figure 1.
Evolution of the Scleractinia since that time some 200 mya is intimately linked with large-scale geo-tectonic movements and related changes in ocean currents. These in turn influenced global climate, along with other intrinsic (biospheric draw-down and release of greenhouse gases) and extrinsic (Milankovitch cycles) drivers.
Of particular importance for scleractinian corals over much of their evolutionary history was the pan-tropical Tethys Sea, separating Gondwana in the south from Laurasia in the north.
Mesozoic
During the Middle and Late Triassic, corals became widespread throughout the Tethys region and their fossils are now found around much of the equatorial Panthalassa Ocean rim. There was a time interval of 20-25 million years between the earliest Triassic corals and the earliest widespread coral reefs. Most noteworthy is that Triassic corals had a wide range of skeletal micro-structures, suggesting that any common ancestry would have been remote. Nevertheless, Triassic corals were not the ecological equivalents of modern corals; corallites were large and poorly integrated so that phacelloid growth forms (where branches are composed of individual corallites) were dominant.
There was a 6-8 million year hiatus between the collapse of Triassic reef development some 202 mya, and the onset of Jurassic reefs, a time of origin of many new scleractinian families. The Late Jurassic was probably the all-time global maximum of Mesozoic coral diversity with at least 150 genera recorded in the European Tethys and 51 in the Panthalassa. Palaeobiogeographic provinces can be recognised which reflect continental plate movements, especially the increasing width of the Protoatlantic. By the Late Jurassic some 150 mya the palaeobiogeographic pattern that had developed was the precursor to the pattern that persisted into the Coenozoic. It was dominated by massive reef development throughout the Tethys, the Atlantic, and also the far eastern Panthalassa. The vast expanse of the eastern Panthalassa was probably a barrier to east-west dispersion, just as the far eastern Pacific is today.

Reconstruction of a Late Triassic coral community. The taxa portrayed may not have occurred in the same geographic region at the same time. The dominant growth forms of the corals are massive and phacelloid. There were no intricately branching corals such are found today and most corals had large corallites. Painting: Geoff Kelly. Figure 2.
A high proportion of the families of extant Scleractinia have their origins in the Middle to Late Jurassic. For most, the fossil record is not clear and thus there are few links between the main branches of the Family Tree. The Jurassic was the time of the proliferation if not origin of two of the most major groups of corals, the Fungiina and the Faviina. The Fungiina dominated much of the Jurassic as well as the Cretaceous. As a group it was greatly diminished by the end-Cretaceous and the families attributed to it today have uncertain affinities. The Faviina, on the other hand, are a well-defined group and the Faviidae have remained a major family for 150 million years.
It is worth noting that various molecular genetic phylogenies of the Scleractinia have been published in recent years. These present a range of evolutionary scenarios for the reef-building and azooxanthellate corals, particularly relations between suborders and families, and remain to be finalized.
Early Cretaceous corals are broadly similar to those of the Late Jurassic. However, relatively little is known of the Middle Cretaceous corals. The continuity of families is largely due to extrapolations between Early and end-Cretaceous fauna, with poorly-known families omitted. This is because, by Middle Cretaceous, reefs worldwide had become dominated by rudist bivalves and environmental perturbations greatly affected reef development. It was not until the very late Cretaceous, following an unexplained total extinction of the rudists that corals returned to a position of dominance. At this time reefs probably again occurred worldwide, but there are few remains of them today.
Coenozoic
One-third of all families and over 70% of all genera became completely extinct at or near the end-Cretaceous boundary some 65 mya. The Faviidae and the Caryophylliidae are the only families that were major components of Mesozoic reefs and that also proliferated in the Coenozoic.
The evolutionary history of modern corals is divisible into three geological intervals (1) the Palaeogene, when the survivors of end-Cretaceous and Late Palaeocene extinctions proliferated into a diverse cosmopolitan fauna, (2) the Miocene, when this fauna became subdivided into the broad biogeographic provinces we have today and pre-cursors of most extant species evolved, and (3) the Plio-Pleistocene to present, when the world went into the series of major glaciations seperated by interglacial periods and modern distribution patterns emerged.
The Miocene is the time of origin of non-Oligocene extant genera (primarily Indo-Pacific) and the immediate ancestors of extant species. It is also the time of obliteration of the Tethys Sea, the extinction of almost all corals from the Mediterranean, and the start of the separate evolutionary histories of Atlantic and Indo-Pacific species.
Compared with most other major groups of animals, coral genera are long-lived in geological time and have low extinction rates: nearly half of all extant genera extend as far back as the Oligocene and nearly one quarter extend back to the Eocene.
The history of corals subsequent to the Miocene becomes decreasingly visible in the fossil record and increasingly visible in the taxonomy and distribution of living corals. The Plio-Pleistocene fossil record of the Caribbean is better than that of the Indo-Pacific and it is in the Caribbean that the impacts of the Pleistocene glaciations were greatest. The progressive closure of the Central American Seaway was one of the most important events in the history of modern corals. Before the closure there may have been no distinction between the corals of the far eastern Pacific and those of the Caribbean. After the closure (3.4 million years ago), the corals of the Pacific side of the Isthmus were extinguished, or nearly so. There are no zooxanthellate scleractinian species common to the Indo-Pacific and the Caribbean today, other than very recent accidental introductions.
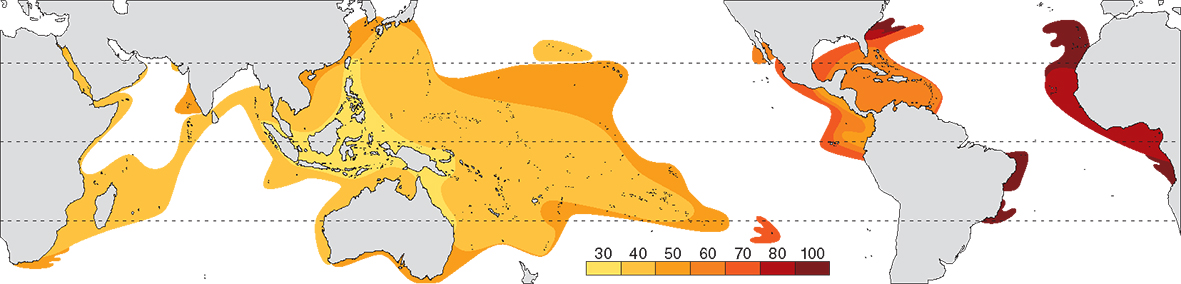
Contours of average age of all extant genera (in millions of years, mya). From Veron (2000a) Corals of the World. Figure 3
The central Indo-Pacific centre of diversity has an average generic age of 30 million years, about half that of the Caribbean. The likely reason for this is that genera that originated in the Tethys Sea (which includes most Caribbean genera) are older than those which originated in the Indo-Pacific, not that evolution has been faster in the Indo-Pacific than elsewhere. The average age of genera in peripheral regions of both the Atlantic and Indo-Pacific is the outcome of a small number of highly dispersed species; it is not created by older genera having more species nor is it created by displacement of species as has been suggested. The peripheral pattern is therefore created by dispersion, not evolution.

Evolution of species
For over two centuries species have widely been regarded as the fundamental units, or ‘building blocks’, of Nature – units that can be named, described and studied. When this concept is applied to corals over large geographic ranges, it breaks down. Thus, a species is only a species in limited geographic space. When that space is progressively increased (as, for example, when studying a particular species in one country, then in progressively more distant countries), it becomes progressively not that species.
The fundamental reason for this is that coral species exist in geographic space as interlinked patterns that change continuously so that variation within a single species becomes indistinguishable from variation between similar species. The majority of species do not exist as geographically or taxonomically definable units. This creates a dilemma, for humans cannot easily communicate in terms of continua: we need discrete units of some form or other. In short, the units we have created in order to communicate are, at best, only an approximation of what actually occurs in Nature.
The issues that arise make it necessary to consider evolutionary change in a way that is different from that which has become generally accepted in both popular and scientific literature. This is ‘reticulate evolution’, a fundamentally distinct concept which involves a different way of looking at what species are, the geographic patterns they make, and their change over evolutionary time. The concept itself is simple, once grasped.
Reconstructing evolutionary change
With corals, as with most other forms of life, evolution cannot be studied directly, it can only be inferred from other studies — palaeontology, taxonomy, biogeography and genetics. The first of these subjects, palaeontology, builds the ‘big picture’ summarised above. It shows something about how life was in the distant past and how it has changed, but it reveals nothing about the mechanisms of change. The last of these subjects, genetics, has the potential to reveal a great deal about evolutionary mechanisms, but this requires vast amounts of study — studies that are only just beginning. To a large extent, biologists depend on taxonomy and biogeography to provide an insight into how species evolve.
Figure 4 illustrates the traditional concept of the phylogeny of species showing:
- species with a time (and therefore place) of origin.
- genetic isolation of phylogenies, once formed.
- an organisation that will conform to a taxonomic hierarchy.
- species that remain distinct: gene pools may become divided, but are not repackaged.
- hybrids (not illustrated) as abnormal units.
- extinction occurring only by termination of lineages.
This phylogeny further implies that the (separately coloured) species are units. If plotted on maps these units can undergo distribution changes, break-up, and go extinct but they will not form other units by lateral transfer of genes. Sympatric and allopatric speciation, reflecting place of origin, are different processes.
Evolutionary change can seldom be observed directly: it must be re-constructed, as is commonly done using family ‘trees’. Family trees are also one way of envisaging reticulate evolution. Such a tree might have a main trunk for the family, several large branches for genera, and many fine branches for the species. Species that are currently alive will be the tips of the uppermost branches and these will be trimmed to uniform height, to represent present time. The branch tips, each representing a single species, can be converted into distribution maps, each map representing the present-day distribution of one species. If a single branch (or species) is then sliced into a sequence of horizontal layers, and each layer is turned into a distribution map, each map will indicate the distribution of the species at progressively distant points in time. If the maps are viewed like the pages of a book, the pattern will change sequentially back in time. These changes will not be just distribution changes, they will also be genetic changes occurring in response to changes in ocean currents. As a result, a species (or map) at one point in time is not the same as it is in another point in time: it has been genetically as well as geographically changed. These changes do not occur uniformly, they occur irregularly over the species’ geographic range and evolutionary history. Geographic space and evolutionary time interact. The species may break apart and then re-form into a slightly different unit. This creates a ‘reticulate’ pattern in both geographic space and evolutionary time.
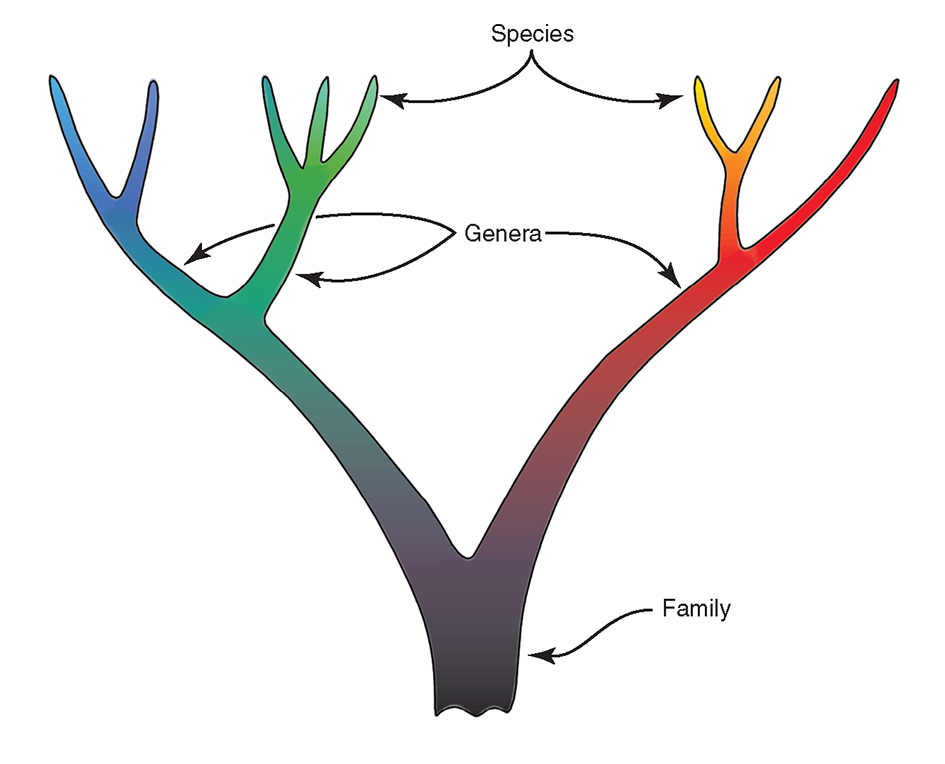
The traditional concept of the species phylogeny. For further explanation see text. From Veron (2000a) Corals of the World. Figure 4
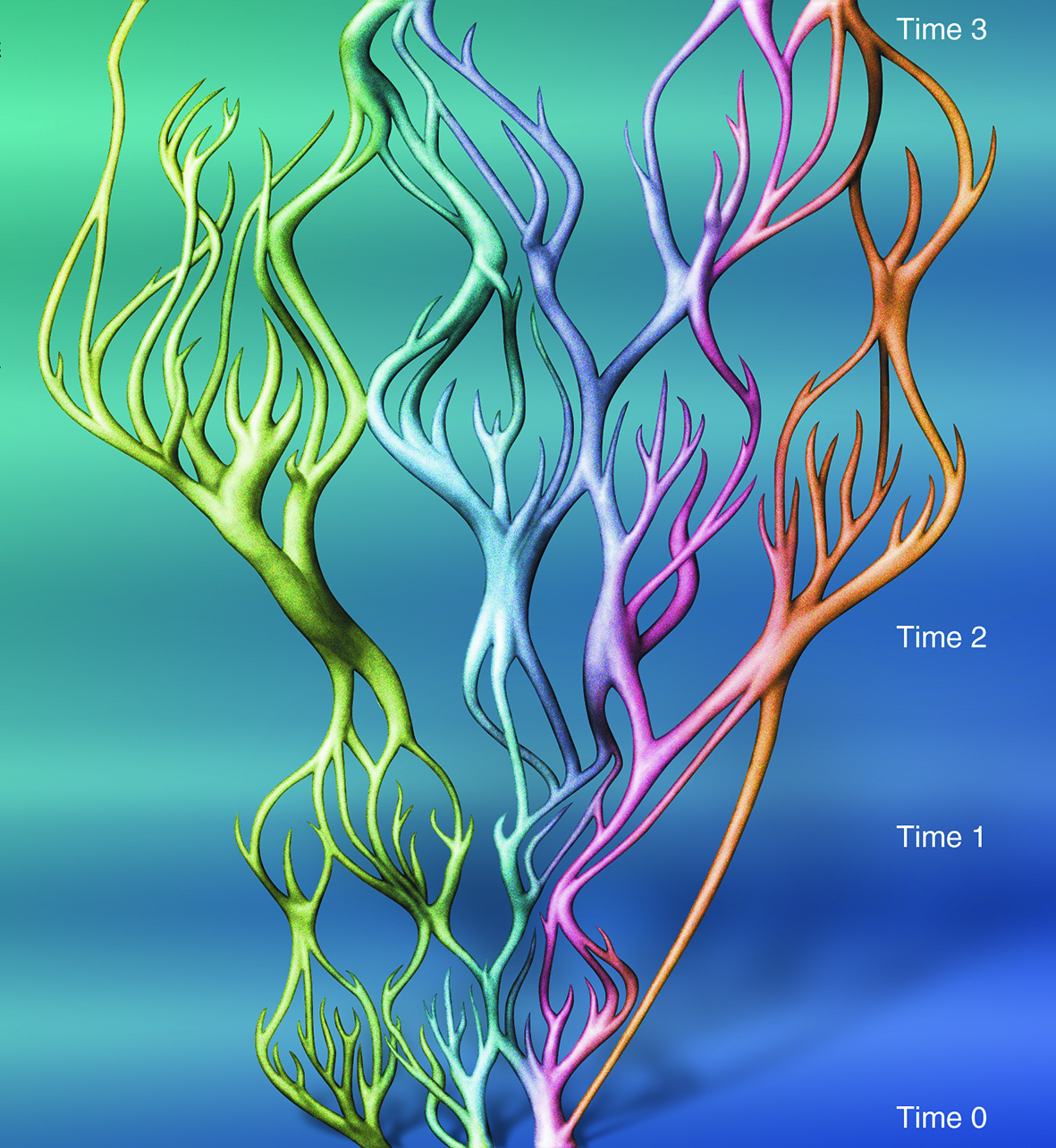
A hypothetical view of reticulate evolutionary change within a group of species. At the bottom (Time 0), the group forms three distinct species, each of which is widely dispersed by strong currents. At Time 1, the group forms many indistinct small species units that are geographically isolated because currents are weak. At Time 2, the group forms three species that are again widely dispersed by strong currents. Over the long geological interval to Time 3, the group has been repackaged several times. For further explanation see text. From Veron (2000a) Corals of the World. Figure 5
Ocean currents and reticulate patterns
When a species breaks apart (as a result of weak ocean currents, see below) it becomes many species. When it re-forms (as a result of strong ocean currents) it may again be a single species or it may be more than one species. It may also contain genes from other species. This is reticulate 're-packaging', and it occurs constantly at all scales of space and time. The re-packaging is not confined to a single phylogeny or evolutionary clade, it involves many clades simultaneously. This is the fundamental reason why traditional cladistics approaches have only limited relevance to understanding the evolution of corals.
Note that in reticulate evolution (see Figure 5):
- no species has a time of origin,
- rates of evolution and extinction are similar over the geological interval represented by the diagram,
- there are no differences between mainstream species (represented by the thicker branches) and subspecies taxonomic levels (represented by thinner branches),
- there are no differences between ‘species’ and ‘hybrids’,
- the total amount of genetic information represented by this diagram has not greatly changed, but has been re-packaged into different ‘species’ units,
- extinction occurs through repackaging as well as terminations of lineages,
- a single species at Time 0 and a single species at Time 3 may have few, if any, morphological distinctions.
Importantly, reticulate evolution is under physical environmental control, not biological control. The physical control changes patterns of genetic connections: it acts on genetic composition. This is again in sharp contrast with a major aspect of the traditional view where evolution is largely controlled by competition between species, resulting in morphological changes. Reticulate evolution is therefore a mechanism of slow arbitrary change rather than a mechanism for progressive improvement.
Reticulate evolution is primarily driven by changes in surface circulation patterns causing changes to the dispersal patterns of larvae. If currents remained constant throughout evolutionary time, the oceans would be divisible into source areas (where larvae come from) and destination areas (where larvae go to) and there would be general uniformity in species and their distribution. However, with the exception of the most major currents, which are driven by the direction of rotation of the Earth, currents are not constant. Sea levels are known to fluctuate over 100 metres, oceanic passages are opened and closed by tectonic movements, and the Earth goes through cyclical climate changes due to variations in the tilt of its axis and variation in the shape of its orbital motion around the sun (Milankovitch cycles). These, and several other types of geo-climatic cycles, cause variations in ocean currents. These changes open and close genetic contacts: they generate reticulate patterns.
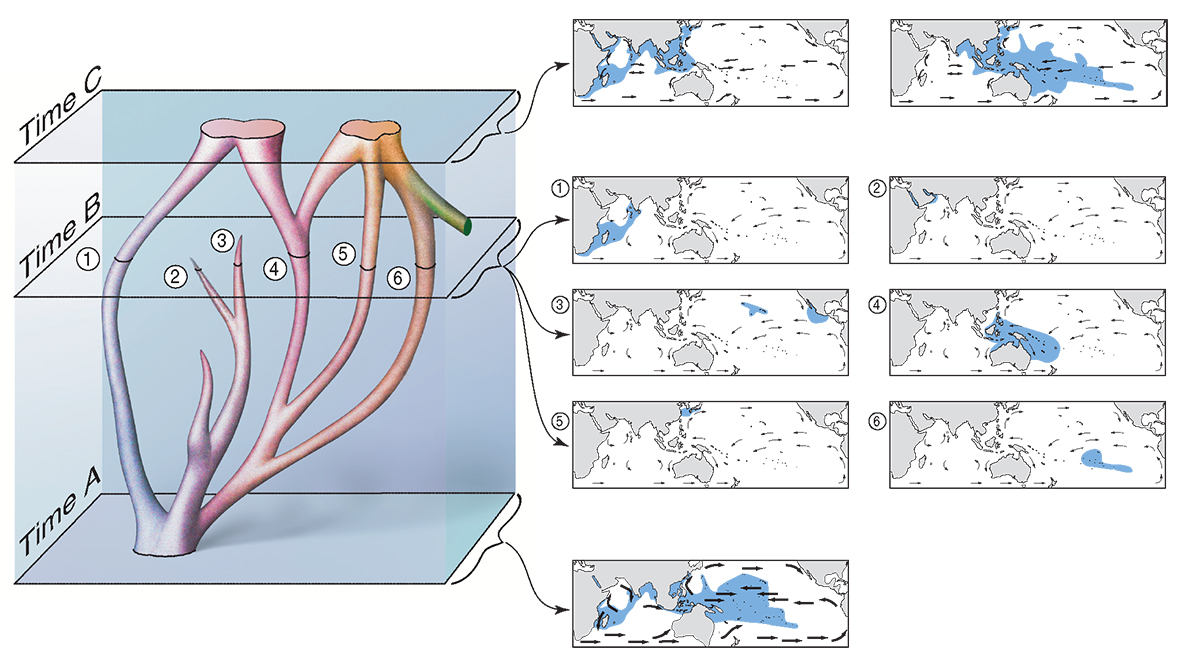
Reticulate evolution and hypothetical distributions observed at various geological time points. A view of evolutionary change in a small phylogeny (left) correlated with hypothetical distribution patterns (maps, right). At the bottom (Time A), currents are strong (and the sea level is low). The phylogeny exists as a single widespread species. At Time B, currents are weak and the phylogeny has broken up into six geographically isolated indistinct or ‘sibling’ species. (Two of these species, labelled 2 and 3, are doomed to subsequent extinction.) At the top (Time 3), currents are strong again. The phylogeny has been re-packaged into two widespread species which are reproductively isolated at this time. The species on the right is part of the phylogeny of another species (coloured green). Note that there is (a) no distinction between geographic (sympatric) and non-geographic (allopatric) origination; (b) evolution is driven by environmental change, not biological competition. From Veron (2000a) Corals of the World. Figure 6.
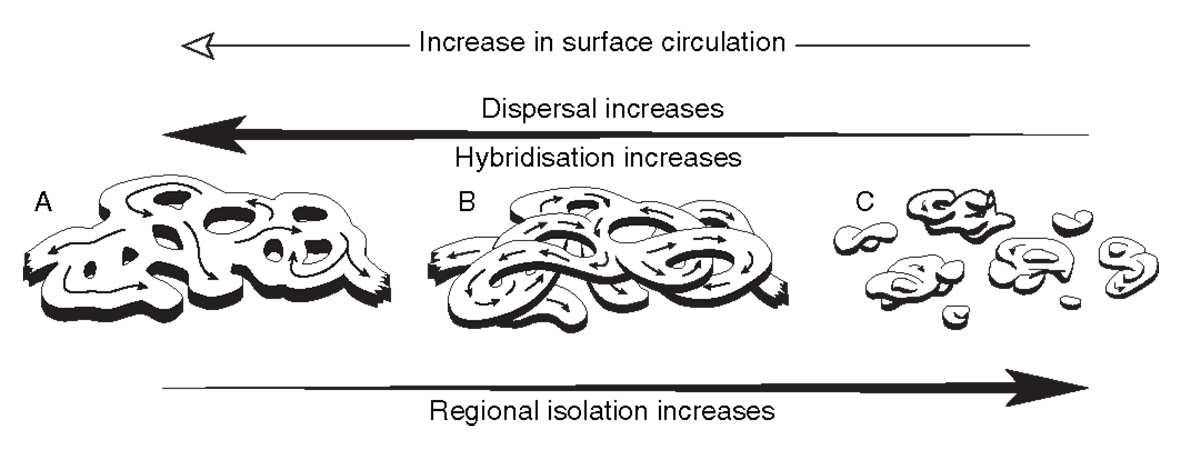
Hypothetical representations of the same gene pool as the figures above under different current regimes. (a) currents are strong and the gene pool forms a single cohesive species, (b) currents are decreasing and the gene pool forms a single species but some parts of it are partly reproductively isolated (represented by overpasses), (c) currents are weak and the species is broken up into isolated pockets. From Veron (2000a) Corals of the World. Figure 7.
To understand how changing ocean currents affect genetic connectivity, it is helpful to imagine what would happen to dispersion if all the ocean currents stopped. Every reef, every island and every headland would be genetically isolated. In time, through the processes of Darwinian natural selection, augmented by genetic drift and mutations, the corals of each location would gradually become distinct from those of every other location. In time, every location would develop a unique fauna and every species would have a distribution range of just one location. There would be millions of species worldwide. If the very opposite is now imagined – where ocean currents are so strong and so variable that the corals of every location came into frequent genetic contact with those of every other location – every species would eventually become dispersed to every location. All species would be found everywhere they could grow and there would only be a small number of species worldwide. It is certain that these imaginary extremes never happened, but what has happened is that the Earth’s climate has oscillated within these extremes, causing constant changes in dispersion, constant changes in genetic connectivity – causing reticulate patterns to arise.
The concept of reticulate evolution is highly explanatory of the observations about coral taxonomy and biogeography and it is also strongly supported by what we know of coral reproduction and is starting to be supported by genetic studies.
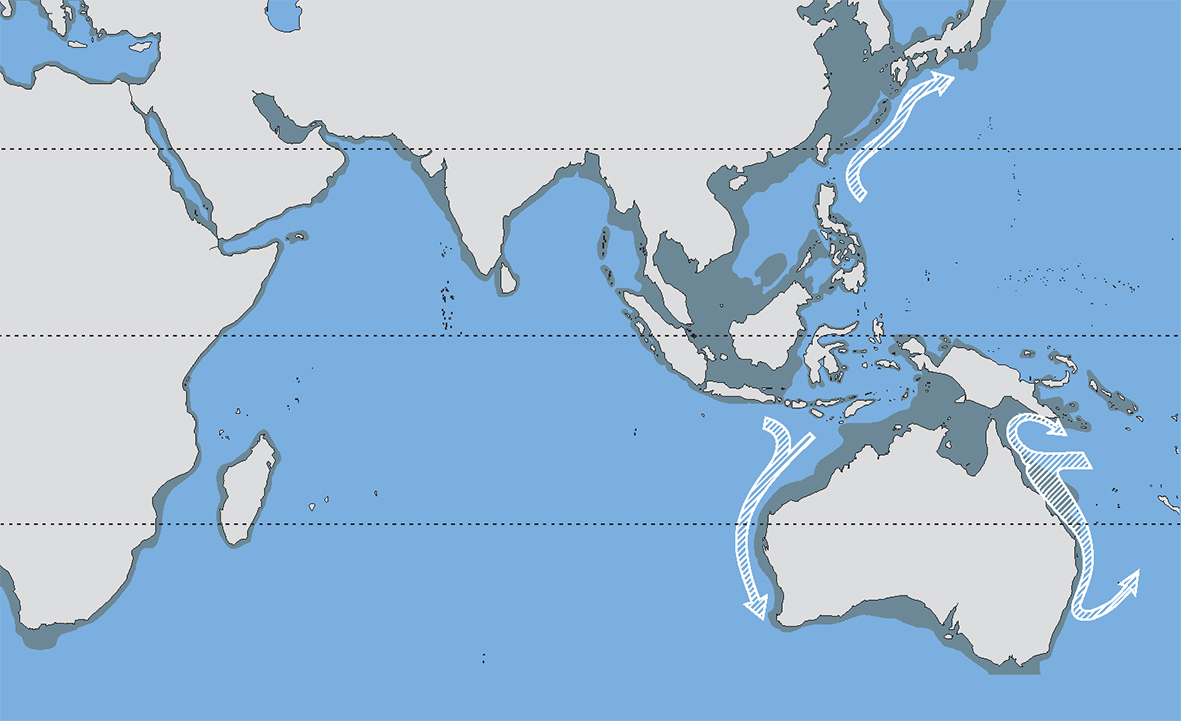
Over the past 18,000 years the coastline of the central Indo-Pacific has moved between the extremes shown here. Over longer intervals of time these sea-level changes have been repeated and combined with substantial tectonic upheavals. These have caused major and repeated changes to the pathways of currents and hence pathways of larval dispersion. Figure 8.
Reticulate and neo-Darwinian evolution
The subject of evolution, now covered by hundreds of books since the epic by Darwin (1859) — On the Origin of Species, is dominated by varying emphases on three broad notions: (a) that species exist in Nature as more-or-less reproductively isolated units of some kind, (b) that these units are comprised of individuals that compete in different ways or are modified by environment and (c) that the ‘fittest’ individuals survive. From these notions there has been an assumption that species have a time and place of origin (where phylogenies divide) and that they are units where geographic space and evolutionary time only interact in a non-reversible manner (where phylogenies divide irreversibly). The concepts of reticulate evolution and the traditional view of evolution are not strongly compatible – they are two paradigms which become increasingly mutually exclusive with increasing amounts of space and time.
There is a point, however, where the paradigm of reticulate evolution interlinks with the traditional Darwinian view of competition and survival. Clearly, coral species compete, perhaps for the same resource (such as food, or living space), or in predator-prey relationships. These sorts of competition affect relative abundance, but only in specific environments, times and places. In other environments, times and places, the dynamics are likely to be different. Thus, one species of coral may continually out-compete another on the east coast of Africa, but this may have little bearing on how they compete on the Great Barrier Reef or how they compete on the east coast of Africa after an environmental upheaval or disease epidemic. For a start, the ‘species’ will not be the same in the two regions. Nevertheless, competition does affect survival. The outcomes will initially be of local significance, but if repeated in many places, they can presumably cause total extinctions. They will not, however, create evolutionary change unless the competing species are, in fact, cohesive units of some kind.
The same issues apply to adaptations to environments: species can adapt to environmental pressures if they are cohesive units of some kind. If any given species is mobile enough to get its genes around the space it occupies in few-enough generations to be able to maintain genetic cohesion, it will be able to adapt as a single unit. Clearly, this can happen with highly mobile organisms like most birds and large mammals. For other organisms, the larger the space they occupy and/or the less mobile they are, the less cohesive they will be. Marine organisms that rely on larval dispersal clearly have minimal chance of cohesion.
It can generally be said that species that are alive today are only those small fragments that have not gone extinct. Ever-changing reticulate systems are not prone to extinction and are resistant to major evolutionary movements. For this reason, evolutionary change in corals large enough to be revealed in the fossil record is extremely slow. At the present point in the Earth’s history, sea levels have been approximately constant for some 7,000 years and there has also been an extended interval of climatic stability. At present, the Earth is likely to be in a period of weak ocean currents that are providing the mechanism for re-packaging species into small units. The stage in a re-packaging sequence reached by each individual ‘species’ will be different as it will depend on genetic dissimilarity with other ‘species’, dispersal ability and abundance. These factors all vary geographically. The decision as to what an individual species is will, in concept, change with evolutionary time, but it will always be arbitrary.


